by James C. Wittig, MD
Chondrosarcoma, although rare, is the second most common bone sarcoma or primary bone cancer. There are several types of chondrosarcomas and each with their own characteristics. Conventional chondrosarcoma is the most common variety. It differs from conventional osteosarcoma and Ewing sarcoma in the fact that it occurs mostly in the adult population in patients greater than 50 years of age. It varies in aggressiveness—rapidity of growth and ability to spread– according to its grade. Most patients diagnosed with chondrosarcoma have pain and/or swelling. Occasionally they will present with a fracture through the bone. Conventional chondrosarcoma is also usually not treated with chemotherapy or radiation since it doesn’t respond to it. This is also another way it differs from conventional osteosarcoma and Ewing sarcoma. They most commonly arise from the pelvis, upper femur, proximal humerus, scapula and ribs. The most common sites of spread (metastatic disease) is to the lungs and other bones).
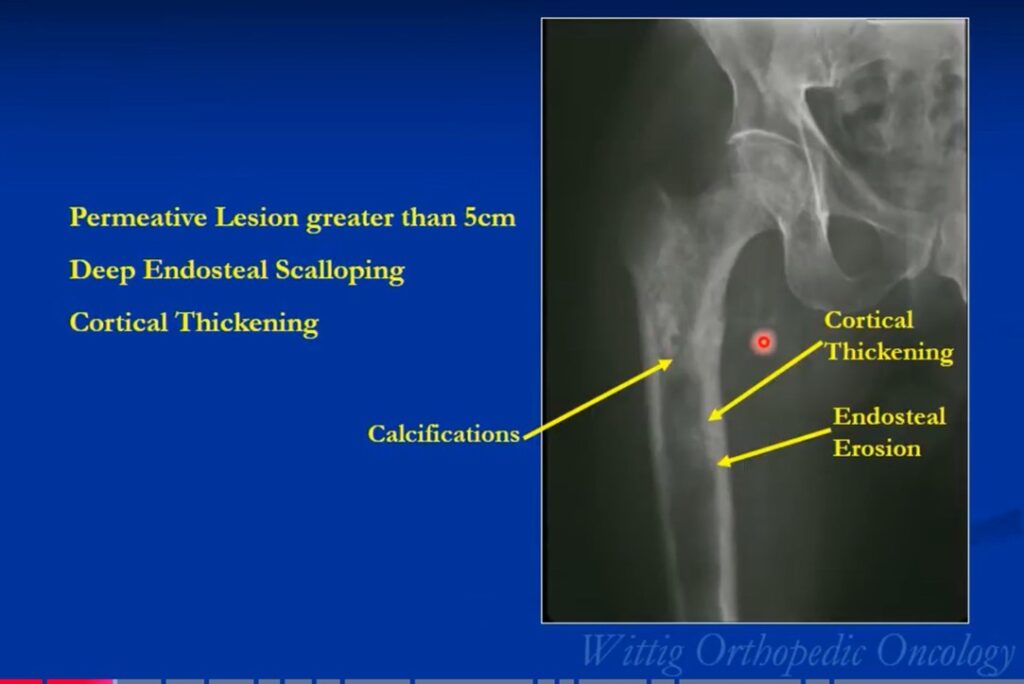
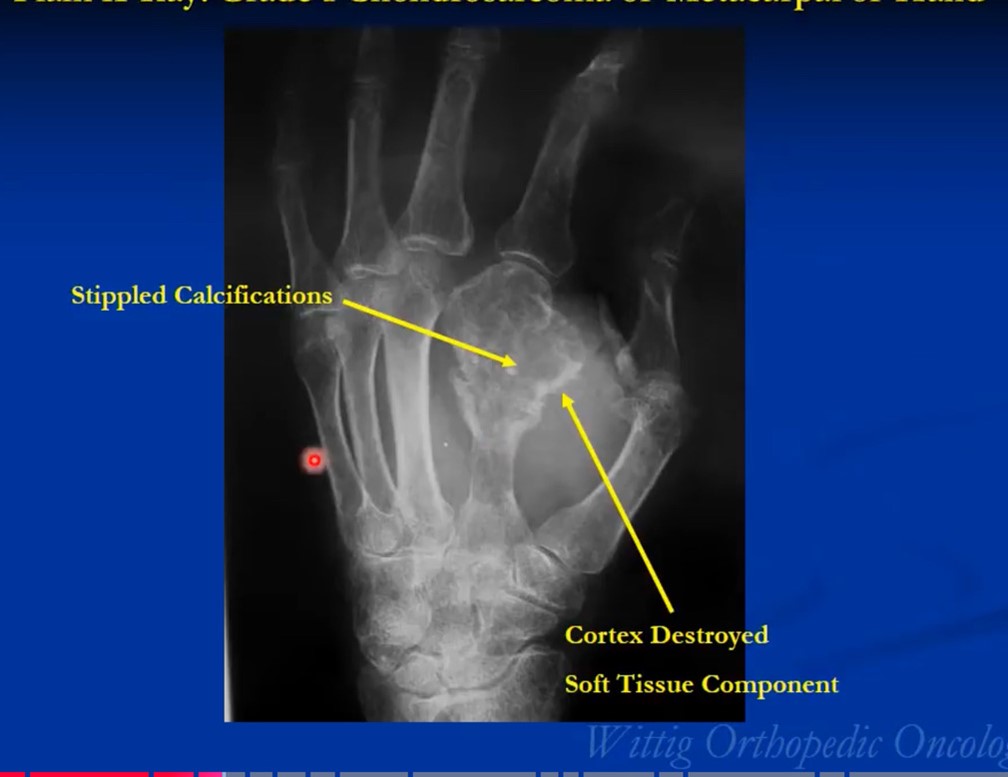
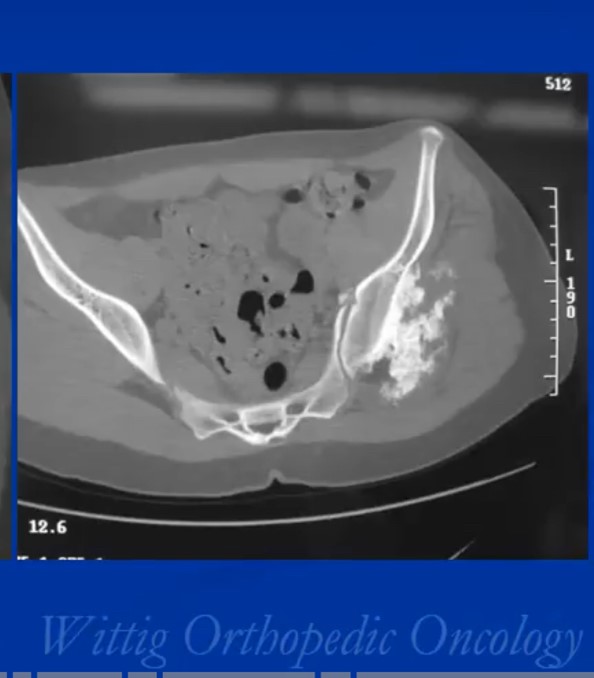
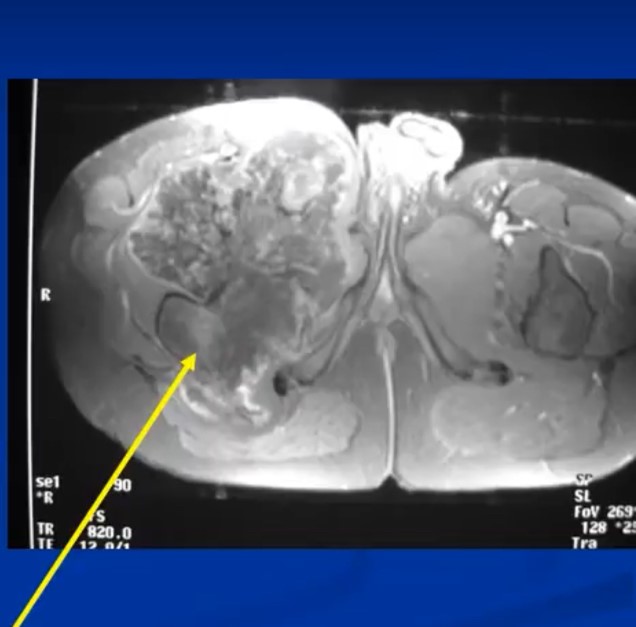
The radiological presentation of a chondrosarcoma depends on its grade. The grade of a tumor indicates how fast the tumor is growing and, hence, its aggressiveness — its ability to invade and destroy adjacent tissues and its potential to metastasize. They are typically graded as Grade I, II or II or low, Intermediate and high grade. Grade 1 or low-grade tumors grow slowly and rarely metastasize. On X-Rays they will often appear well circumscribed and contain calcifications in a ring and arc like manner. Popcorn like or stippled calcifications are characteristic for diagnosis of cartilage tumors. They may be entirely intraosseous and usually greater than 5 cm in length. There may be cortical thickening or deep endosteal scalloping. Rarely does one penetrate the cortex. In over 50% of cases, they are hotter than the anterior superior iliac spine on a bone scan. Grade 3 or high-grade tumors grow rapidly and spread readily. They are usually permeative on an Xray and not circumscribed. They are often associated with a soft tissue mass outside the bone and may have sparse calcifications or there may be areas with heavy calcifications adjacent to areas where it appears calcifications have been resorbed. There may be cortical thickening and periosteal reactions. CT scans may be useful for detecting subtle calcifications and cortical destruction. MRI is useful for demonstrating lobular growth as cartilage tumors grow in lobules and for showing the extent of the tumor. Diagnosis is primarily made with X-rays and a biopsy. Conventional Chondrosarcoma can arise from an osteochondromas. It is a rare event. Most are low grade and characterized by a thickened cartilaginous cap greater than 2 cm on an MRI.
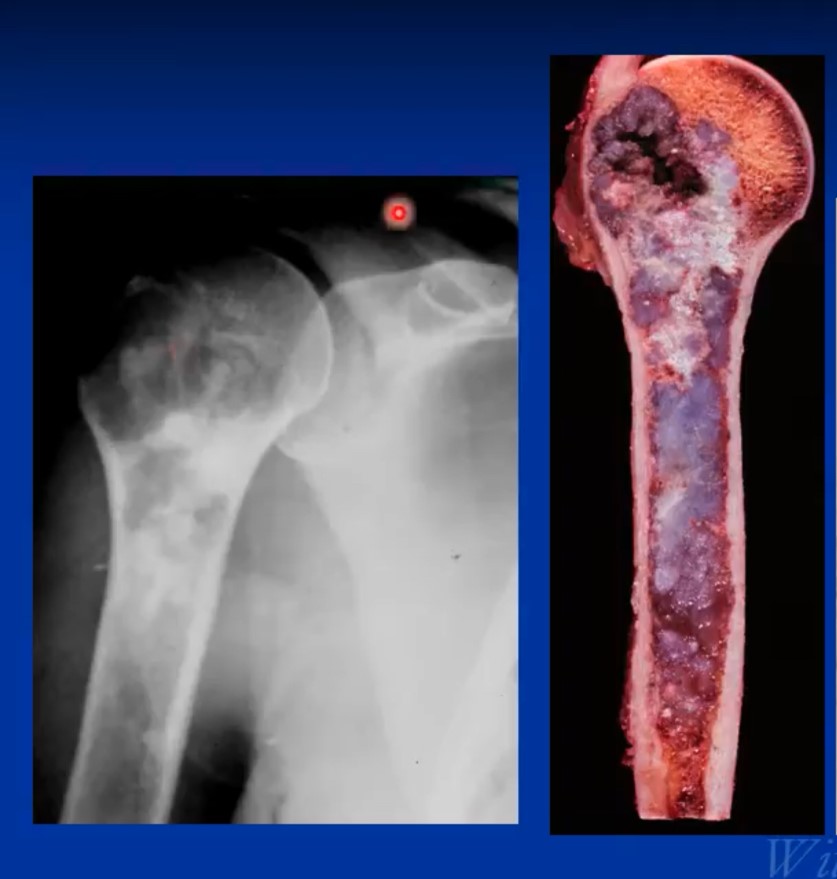
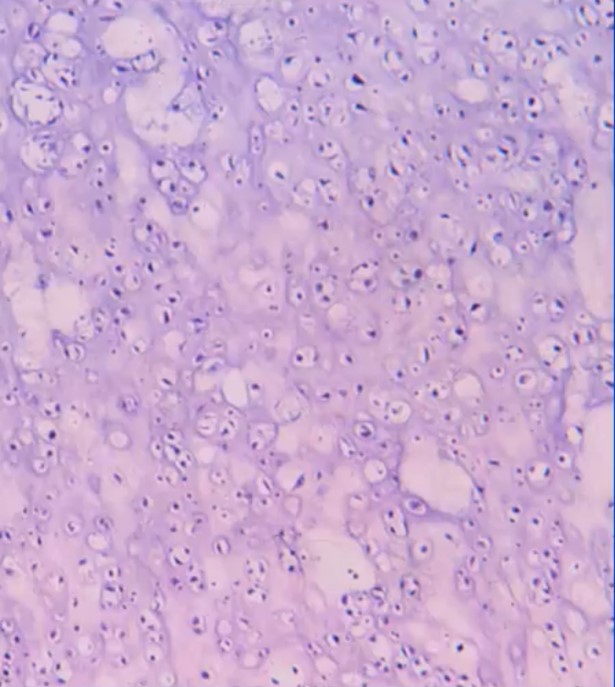
The pathological or microscopic features of a conventional chondrosarcoma emulate the radiological appearance. Hyaline cartilage tumors grow in a lobular manner and have cells in lacunae within a ground glass or cartilage appearing matrix. Grade 1 or low-grade tumors are hypocellular and have a significant amount of matrix. There is usually more matrix than cells and the cells are usually more uniform and laid down more orderly. There are minimal to no mitotic figures and no necrosis. Identifying bone entrapment where the cartilage surrounds trabeculae of bone is the key to diagnosing it as a malignancy. As grade increases from grade 1 to grade 3 or low-grade show growing to faster growing and higher grade, the cellularity increases as well as the percentage of multinucleated cells/multiple cells in lacunae, mitotic figures size and shape of cells. The cells become less orderly and in grade 3 tumors may spindle and may barely resemble typical cartilage cells.
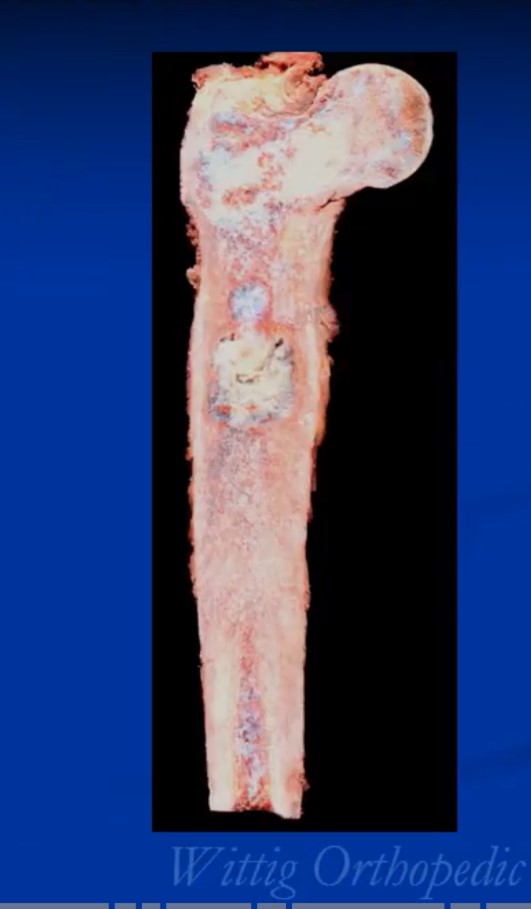
Treatment of conventional chondrosarcoma consists of surgery alone. Surgical treatment depends on grade and extent. Most grade 1 chondrosarcomas and some grade 2 tumors that are entirely intraosseous may be considered for treatment with an intralesional curettage resection and cemented fixation. An adjuvant such as cryosurgery may also be considered. Occasionally a radical limb sparing resection and reconstruction may be necessary for grade 1 tumors. Grade 3 chondrosarcomas and many if not most grade 2 chondrosarcomas are treated with a radical resection and reconstruction. Chemotherapy is controversial as its never been shown to be effective offer a survival benefit but some patients depending on many factors and if they have a high-grade tumor may be recommended chemotherapy or other systemic treatments. Radiation is also typically not effective but may be recommended in selected situations for large pelvic tumors where margins may be close after resection. In most cases, limb saving surgery can be performed. Occasionally there may be indications for an amputation. It may be more difficult to salvage patients who develop fractures through their chondrosarcomas compared to osteosarcoma and Ewing sarcoma since chondrosarcoma does not respond to chemotherapy like the other two common bone sarcomas. When a pathological fracture through the tumor happens, the bleeding spreads the cells into the surrounding tissues making it difficult to save the limbs especially for tumors that don’t respond to radiation or chemotherapy as these adjuvant treatments are often used in conjunction with surgery to prevent microscopic disease from recurring.
Prognosis most strongly depends on if the tumor has already spread at the time of presentation. Patients with overt metastatic spread have approximately 15% 5-year survival rates if the primary tumor can be removed along with the metastatic disease. In patients who present without overt metastatic spread, prognosis depends on grade and location. Pelvic tumors have a worse prognosis. Grade 3 tumors have a far worse prognosis than grade 1 tumors. Almost all patients with grade 1 chondrosarcoma are cured while grade 3 tumors are associated with approximately 65% 5-year survival. Grade 3 tumors in the pelvis have a worse survival rate with approximately 55% of patients surviving 5 years after successful resection.
Latest Studies
Here’s what the literature shows from the last ~10 years (2015–2025). There are essentially no classic, head-to-head randomized controlled trials (RCTs) in chondrosarcoma that demonstrate a survival benefit. The lone randomized, placebo-controlled study (regorafenib) was non-comparative by design and did not meet its primary endpoint, though it suggested a PFS signal.
10 peer-reviewed studies from 2015-2025
- Regorafenib vs placebo (non-comparative, randomized, double-blind phase II) — EORTC/FSF (2014–2019)
- Population: 46 pts with metastatic/locally advanced chondrosarcoma progressing after therapy.
- Intervention: Regorafenib vs placebo, 2:1; cross-over allowed.
- Primary endpoint: 12-wk progression-free rate (PFR).
- Key result: 12-wk PFR 54.2% on regorafenib vs 31.3% on placebo; median PFS 19.9 vs 8.0 weeks; trial did not meet its primary statistical success threshold. Safety acceptable but included one treatment-related fatal liver dysfunction. PubMed
- Pazopanib single-arm phase II (City of Hope multi-center)
- Population: 47 pts with unresectable/metastatic conventional chondrosarcoma.
- Primary endpoint: Disease control at 16 wks.
- Key result: 16-wk DCR 43% (above predefined null), median PFS 7.9 mo, median OS 17.6 mo; 1 partial response. PubMed
- Ivosidenib (IDH1 inhibitor) phase I — initial report (JCO 2020)
- Population: 21 pts with IDH1-mutant chondrosarcoma.
- Key result: Plasma 2-HG fell in all; median PFS 5.6 mo; 6-mo PFS rate 39.5%; disease control in ~52%; minimal toxicity. PubMedASCOPubsMD Anderson Cancer Center
- Ivosidenib (IDH1) phase I — long-term update (Clin Cancer Res 2025)
- Population: Same program with extended follow-up; 13 conventional CS in analysis.
- Key result: For conventional CS, ORR 23.1%; median PFS 7.4 mo; several pts on therapy >2–7 years; manageable toxicity. (Ongoing phase 3 CHONQUER randomized placebo-controlled study.) PubMedAACR Journalsserviermedical.us
- INBRX-109 (ozekibart; DR5 agonist antibody) phase I (Clin Cancer Res 2023)
- Population: Unresectable/metastatic chondrosarcoma cohorts.
- Key result: Disease control 87%, durable clinical benefit ~41%, median PFS 7.6 mo; mostly low-grade AEs. Randomized, placebo-controlled phase II (ChonDRAgon) ongoing. PubMed
- Pembrolizumab (SARC028) — bone sarcoma cohort (Lancet Oncology 2017)
- Design: Two-cohort, single-arm phase II.
- Chondrosarcoma subset: 1/5 (20%) objective response; overall bone-sarcoma ORR 5%. Primary endpoint not met in cohorts, but activity signal in select histologies. PubMed
- Apatinib (VEGFR-2 TKI) — two-center retrospective series (Cancer Management & Research 2020)
- Carbon-ion radiotherapy (CIRT) — extracranial CS initial experience (J Cancer 2019)
- Population: 21 pts total; 5 chondrosarcoma (with chordoma cohort).
- Key result: Favorable short-term LC/PFS/OS with no severe acute/late AEs; authors call for prospective randomized evaluation. jcancer.org
- High-dose proton therapy (PT) for skull-base chordoma/chondrosarcoma — prospective series
- Design / Sites: Early prospective data show PT (alone or with IMRT) feasible with encouraging LC/toxicity; includes CS subset. (e.g., Dastgheyb et al., 2024; Beaumont initial results 2021). ScienceDirectCureus
- Hypofractionated 5-fraction proton therapy — early prospective trial framework (Cancers 2023)
- Population: Skull-base chordomas & chondrosarcomas; early results + detailed trial design.
- Key impression: Feasible hypofractionation with promising early LC and tolerance; longer follow-up pending. MDPI
Related prognostic evidence (not interventional but often asked): IDH1/2 mutation status is common and its prognostic impact is mixed across studies—some show worse survival with IDH1 R132, others show no association; results are inconsistent and may depend on grade and cohort composition. Lippincott JournalsPMCMDPI
Take-aways on RCTs & survival benefit
- Published RCTs specific to chondrosarcoma are virtually absent. The regorafenib study is randomized and placebo-controlled but non-comparative and did not meet its primary success criterion (though median PFS almost doubled vs placebo and 12-wk PFR favored regorafenib). No OS advantage has been proven in any RCT. PubMed
- Signals worth watching: IDH-targeting (ivosidenib) and apoptosis-targeting (INBRX-109) produced multi-month median PFS and durable disease control in subsets. Phase 2/3 randomized trials are underway, but no peer-reviewed survival-positive randomized data yet. serviermedical.usPubMed
- Anti-angiogenics (pazopanib, regorafenib, apatinib, anlotinib-based programs) show modest PFS/DCR benefits in small/early studies. PubMed+2PubMed+2
- Proton/carbon-ion radiotherapy offers high local control with acceptable toxicity in skull-base or selected extracranial CS, informing prognosis after local therapy; these are not randomized head-to-head vs photons. ScienceDirectjcancer.orgMDPI
Full references
- Duffaud F, et al. Eur J Cancer. 2021;150:108-118. “Efficacy and safety of regorafenib… chondrosarcoma” (randomized, double-blind, placebo-controlled, non-comparative phase II). PubMed
- Chow W, et al. Cancer. 2020;126:105-111. “Prospective phase 2 pazopanib in unresectable/metastatic conventional chondrosarcoma.” PubMed
- Tap WD, et al. J Clin Oncol. 2020. “Phase I ivosidenib in IDH1-mutant chondrosarcoma.” PubMed
- Tap WD, et al. Clin Cancer Res. 2025;31:2108–. “Phase I ivosidenib long-term safety & activity in conventional chondrosarcoma” (+ CHONQUER phase 3 overview). PubMedAACR Journalsserviermedical.us
- Subbiah V, et al. Clin Cancer Res. 2023;29:2988-3003. “INBRX-109 (DR5 agonist) preclinical + phase I in chondrosarcoma.” PubMed
- Tawbi HA, et al. Lancet Oncol. 2017;18:1493-1501. “SARC028 pembrolizumab bone cohort (includes chondrosarcoma).” PubMed
- Xie L, et al. Cancer Manag Res. 2020;12:—. “Apatinib for inoperable metastatic/locally advanced chondrosarcoma (two-center retrospective).” PubMedPMC
- Wu S, et al. J Cancer. 2019;10:3315-3322. “Carbon Ion Radiotherapy for extracranial chordoma & chondrosarcoma — initial experience.” jcancer.org
- Dastgheyb SS, et al. Adv Radiat Oncol. 2024. “Prospective high-dose proton therapy for chordomas and chondrosarcomas.” ScienceDirect
- Sallabanda M, et al. Cancers (Basel). 2023;15:5579. “Five-fraction proton therapy — early prospective results & trial design for skull-base chordoma/CS.” MDPI
Practical summary for clinic
- Systemic therapy: Consider pazopanib or regorafenib in progressive metastatic conventional CS; IDH1-mutant pts may be candidates for ivosidenib (on-trial if possible); INBRX-109 looks promising (seek trial). Immunotherapy has limited but occasional activity (rare responses). PubMed+4PubMed+4PubMed+4
- Local therapy/prognosis: For skull-base/selected sites, proton or carbon-ion RT can yield strong local control with acceptable toxicity—key for long-term outcomes when surgery is morbid or incomplete. ScienceDirectjcancer.org